Here’s a quick breakdown of what you’ll learn in this blog:
- Client Trust in In Vivo CROs: How accurate in vivo study design builds client confidence, reduces variability, and ensures reproducibility needed for long-term relationships
- Preclinical CRO Challenges: Understanding variability in in vivo studies, outdated protocols, and balancing innovation with reliability in preclinical contract research
- AI-Powered In Vivo Study Design: How AI tools and best practices enable in vivo CROs to accelerate study design, improve accuracy, and enhance client satisfaction.
In Vivo CROs and Preclinical Excellence
Preclinical contract research organizations (CROs) that specialize in in vivo studies play a pivotal role in guiding pharmaceutical clients through complex preclinical drug development. Clients place immense trust and financial investment in in vivo CROs, expecting rigorous, cutting-edge in vivo study designs that maximize therapeutic potential.
As experimental design scrutiny increases, CROs must demonstrate expertise and reliability to build enduring client relationships.
This blog examines the challenges faced by in vivo CROs, highlights how innovative AI-powered approaches enhance in vivo preclinical research, and explores how precision in in vivo study design strengthens client trust in CROs.
The Critical Importance of Client Trust for In Vivo CROs
CROs bear unique responsibility in preclinical drug development because in vivo studies directly assess how drugs behave in living organisms, a critical step before human trials. Regardless of reputation, clients must place fundamental trust in the organization to execute in vivo experiments to the highest standards, thoroughly exploring every avenue to give their novel therapy the best possible chance of success.
Although clients outsource many essential tasks to, this does not imply a lack of understanding of good experimental design on their part. Rather, preclinical CROs are often utilized as collaborative partners to fast-track studies, provide access to advanced technologies, and offer expertise on later-stage clinical trials. Considering the significant investment and high stakes involved in engaging an for preclinical work, clients carefully scrutinize in vivo experimental designs to ensure they are receiving value for their money and confirm that they are competent and capable.
Effective in vivo experimental design enables them demonstrate they fully understand client goals and know the best strategies to achieve them. It also reassures clients that the they can reliably manage the later stages of clinical trials, especially for discovery-focused clients less familiar with these complex stages.
Longitudinal success is built on reputation and trust. Expectations for quality and efficiency continue to rise for both biotech companies and CROs specializing in in vivo studies. Modern AI-driven solutions for in vivo study design allow CROs to instantly scan entire literature databases to identify optimal parameters for in vivo work.
These tools empower them not only to convey authority and expertise but also to back it up with reliable data within timeframes that meet client expectations. Just as automated platforms revolutionized high-throughput screening, innovative approaches to in vivo study design transform how to develop experiments, enabling faster and more precise protocol development. CROs specializing in in vivo work can no longer overlook opportunities for increased efficiency.
Key Challenges Faced in Building Client Confidence
Preclinical in vivo CROs face distinct challenges that significantly impact their ability to attract new clients, build lasting partnerships, and establish themselves as reliable partners for in vivo preclinical research.
Variability in In Vivo Study Results
Variability in in vivo study results represents a significant barrier for to demonstrate reliability and consistency. While variation is inherent to biological science, it's particularly apparent in in vivo work, where animals are chosen to represent human biological complexity. However, this similarity also increases the chances of variation between individuals, which can reduce study reproducibility if not properly accounted for.
This reproducibility challenge is now widely recognized across biological research. A landmark study found that results from just 23 of 53 prominent cancer biology papers could be replicated by independent researchers. Although numerous sources of variability exist in in vivo experimentation, responsibility often falls on the researchers conducting the experiments.
While some variability is beyond investigator control, in vivo CROs must do everything possible to ensure variables are controlled wherever feasible. This comes down to detailed, thorough experimental design specific to each in vivo study.
Limitations of Traditional In Vivo Study Design Approaches
Study design often suffers from overreliance on outdated protocols, which may produce worse results than more modern but less-cited methods. Academic laboratories are particularly prone to relying on inherited protocols, but preclinical CROs have an increased obligation to ensure they are performing optimal in vivo experiments based on current gold standards.
The rise of modern tools for in vivo study design, coupled with a drive toward increased transparency in preclinical research, ensures that unjustifiable rigidity and reluctance to adopt contemporary in vivo approaches will not go unnoticed by research-savvy clients. Clients familiar with the latest developments in their field have a good grasp of available in vivo models and expect their in vivo CRO partner to leverage them.
Poorly designed in vivo experiments result in poor outcomes. When clients are selecting an partner, experimental design quality is often their first evaluation criterion. Thus, in vivo CROs must keep a finger on the pulse of emerging in vivo models and standards to maintain client trust.
Competitive Landscape and Innovation Expectations
Preclinical in vivo CROs must strike a balance between reliability and innovation to differentiate themselves in an increasingly competitive landscape. There are many options available for performing in vivo experiments, which can sometimes be more of a hindrance than an advantage. The abundance of in vivo methodologies makes it virtually impossible to gain a full grasp of the in vivo experimental design landscape, making it difficult to gauge client expectations.
Additionally, adopting new in vivo approaches solely for the sake of innovation can backfire, earning a reputation for recklessness. Conversely, being overly cautious might leave clients feeling they are not maximizing their investment in in vivo studies, potentially causing key in vivo parameters that might provide valuable insights for advancing to human trials, to be overlooked.
Successfully balancing innovation with success requires a broad and deep understanding of in vivo literature. Strike this balance and earn reputations for forward-thinking coupled with research excellence.
How Precision in In Vivo Study Design Builds Customer Trust
Good in vivo study design communicates research excellence, but ultimately results determine whether an in vivo CRO earns client trust. Accurate in vivo study design is crucial for avoiding errors, meeting timelines, and establishing reproducibility across in vivo research.
The Preclinical In Vivo Drug Development Process
The preclinical drug development process involves establishing a drug's safety, efficacy, and feasibility before progressing to human trials. In vivo studies are particularly critical in this stage. The process begins with target identification and validation, followed by lead compound optimization.
In vivo studies and complementary in vitro studies are conducted to evaluate biological activity, pharmacodynamics, and pharmacokinetics.
In vivo toxicology tests assess acute, chronic, and reproductive toxicity.
Pharmacokinetic and pharmacodynamic studies conducted in vivo further explore absorption, distribution, metabolism, and dose-response relationships.
Once these in vivo parameters are satisfactorily addressed, manufacturing processes are scaled up in compliance with Good Manufacturing Practices (GMP). Preclinical in vivo data are then submitted to regulatory agencies as part of an Investigational New Drug (IND) application. Upon IND approval, the drug proceeds to clinical trials.
How Precision Reduces Variability and Errors in In Vivo Research
Choosing every in vivo study parameter carefully and with specific outcomes in mind is essential but challenging for in vivo CROs working with short timelines and information scattered across literature databases. However, understanding the role of each in vivo parameter and the ramifications of different in vivo options is essential for reducing variability and errors in in vivo studies.
Errors often arise where in vivo protocols are unclear or when sections are copied from preexisting in vivo protocols that may not be fully compatible with the current study. Careful in vivo experimental design minimizes the impact of biological variability on in vivo study results. A well-planned in vivo approach ensures selection of the most suitable animal model and the most effective readouts to answer the research question accurately.
Sample size is another critical area where inadequate rigor in in vivo studies causes problems. Insufficient sample sizes in in vivo experiments may result in lack of statistical power, while excessive in vivo sample sizes waste resources and negatively impact animal welfare.
Establishing Robust Reproducibility in In Vivo Research
Many factors enhance reproducibility in in vivo research. To improve in vivo reproducibility, focus on:
- Establishing blinding and randomization in in vivo study groups
- Ensuring clearly defined hypotheses and goals before in vivo experiments begin
- Including adequate in vivo control groups to draw reliable conclusions
- Enabling others to effectively replicate in vivo results
Modernizing In Vivo Study Design: Advanced Solutions for Excellence
Many modern tools are emerging that remove significant risks from in vivo experimental design, making it simpler to perform robust in vivo studies that align with client expectations.
Cutting-Edge In Vivo Technologies and AI Solutions
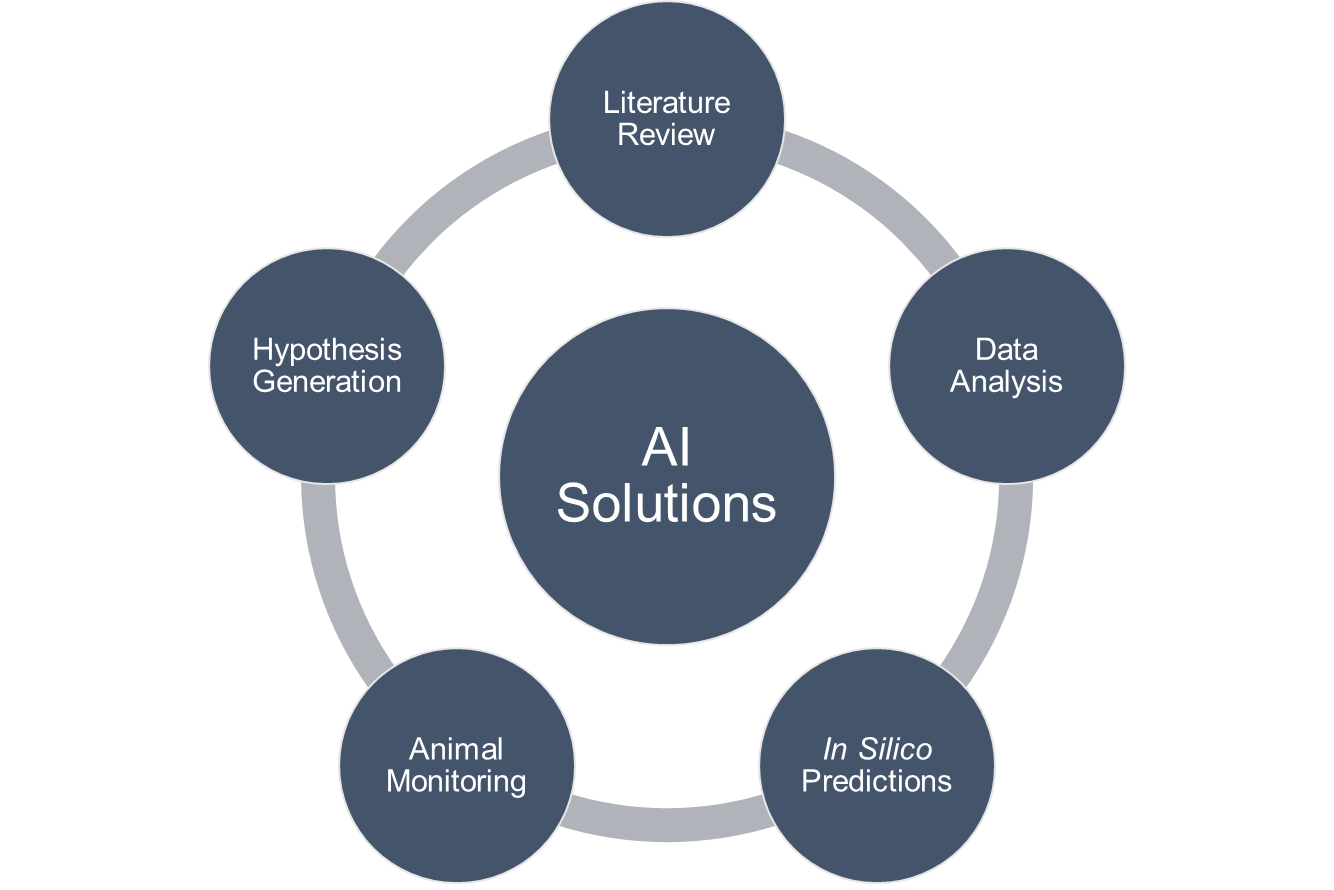
AI and machine learning are transforming in vivo experimental design. These technologies are being applied across several in vivo research areas:
- Virtual Screening: AI performs virtual screening of compounds for in vivo toxicology studies, simplifying and refining in vivo experimental setups while facilitating dose optimization
- Pharmacodynamics/Pharmacokinetics Prediction: AI can predict PD/PK of different therapeutics, particularly valuable for simulating newer biologics-based treatments in in vivo models
- Literature Analysis: AI platforms scan and summarize available approaches to in vivo research questions, allowing in vivo CROs to quickly identify optimal parameters without manually reviewing vast in vivo literature databases
- Experimental Planning: AI agents can integrate data with in vivo simulations, designing in vivo experiments and guiding discovery toward high-impact in vivo research opportunities
Advanced Data Analytics: AI performs advanced analysis across in vivo datasets, single-cell omics, microscopy, spatial biology, viability experiments, giving in vivo researchers clarity on variables affecting in vivo results
Key Benefits of Precision In Vivo Experimental Design
Confidence in In Vivo Research Outcomes
While researchers may not always achieve desired results from in vivo experiments, a well-designed in vivo study remains valuable by providing sufficient information to support downstream decision-making. Precision in vivo tools enhance researchers' confidence in in vivo results by ensuring in vivo experiments are properly designed to minimize confounding variables.
Pursuing clinical research is inherently risky and costly. Phase III trial failures often result from lack of robustness at the preclinical in vivo stage. Well-designed in vivo studies provide CRO clients with stronger evidence to convince investors that their potential therapy has higher likelihood of success.
Conversely, if in vivo results indicate the therapy should not proceed to clinical trials, researchers can use the in vivo data to refine their next therapeutic candidate. This approach avoids investing in therapies likely to fail in human trials while equipping researchers with valuable insights. Poor in vivo experimental design can leave researchers uncertain how to proceed or whether to proceed at all.
Faster Timelines Through Optimized In Vivo Design
Precision in vivo design helps researchers stay on schedule. Well-designed in vivo experiments require minimal adjustments, ensuring efficient paths to results. Advanced AI-assisted tools for in vivo design not only lead to more accurate in vivo protocols with greater success probability but also enable streamlined in vivo design processes. Researchers don't need to spend months devising in vivo protocols; AI tools allow them to quickly pull the most relevant in vivo information from diverse sources, immediately outpacing manual in vivo design methods.
Better in vivo design allows researchers to align with industry trends. Using the latest and most advanced in vivo models conveys professionalism and authority to reviewers and makes it easier for regulators to understand in vivo protocols, giving therapies the best chance of success.
Best Practices for In Vivo CROs Implementing Precision and Modernization
With many AI-assisted solutions on the market, CROs need strategic approaches for implementing these in vivo tools effectively. While AI makes in vivo processes more efficient, they must establish systematic approaches for in vivo experimental design, personnel training, and client communication.
Establishing a Systematic In Vivo Experimental Design Approach
In vivo experimental design can be overwhelming given the numerous in vivo parameters requiring careful consideration within restrictive budgets and time constraints. In vivo CROs must balance thoroughness and accuracy with practical resource limitations while ensuring compliance with ethical standards and in vivo regulatory requirements.
An important first step is achieving alignment with the sponsor on a concise in vivo hypothesis to be tested. A clear in vivo hypothesis makes all subsequent in vivo design decisions far more straightforward and is particularly important when using AI-driven tools for in vivo design. After the in vivo hypothesis is agreed upon, identifying the best in vivo model should become the primary aim. Other in vivo study parameters should be selected to facilitate this in vivo model.
Training for In Vivo Preclinical Researchers
AI tools can establish in vivo hypotheses and identify optimal in vivo models and in vivo parameters. However, personnel using these in vivo tools must receive proper training.
Most AI tools for in vivo research are user-friendly, but collaborating with in vivo platform providers ensures smoother transitions. Clear communication about why new in vivo approaches are being implemented and how this impacts daily in vivo operations is essential.
Client Engagement and In Vivo Transparency
AI-based in vivo tools are becoming more widely used, but hesitation to implement these in vivo technologies is understandable for high-stakes applications like in vivo experiments. AI suffers from the "black box" problem where users don't understand how AI generates in vivo recommendations, leading to concerns about in vivo transparency, reliability, and accountability.
This lack of interpretability can make in vivo researchers wary of trusting AI-generated in vivo recommendations. The concern is amplified when CRO researchers use AI to design in vivo experiments for clients. Being transparent about how AI is used in in vivo design and allowing questions and feedback are essential for helping clients understand and accept modern in vivo approaches.
Highlighting how AI addresses specific client concerns, such as in vivo regulatory compliance or animal welfare, and showcasing its strengths, such as employing innovative in vivo methodologies, can effectively gain client support.
Conclusion: In Vivo CROs Leading Preclinical Excellence
Precision-driven and modernized in vivo study design enhance client confidence, mitigate in vivo variability, and align with stringent in vivo regulatory standards while accelerating project timelines. As the preclinical in vivo research landscape evolves, leveraging cutting-edge tools like AI-driven in vivo design platforms is no longer optional but essential for staying competitive.
Embracing precision in in vivo experimental design and modern in vivo technologies position themselves as vital partners in advancing preclinical research excellence. By implementing systematic approaches to in vivo study design, investing in in vivo researcher training, and maintaining transparency with clients about in vivo methodologies, in vivo CROs can drive transformative breakthroughs while reinforcing client trust.
Ready to transform your in vivo CRO capabilities?
Get Your ModernVivo Demo Today!
References
1. Lane RF, Friedman LG, Keith C, et al. Optimizing the use of CROs by academia and small companies. Nat Rev Drug Discov. 2013;12(7):487-488. doi:10.1038/nrd4057
2. Von Kortzfleisch VT, Karp NA, Palme R, Kaiser S, Sachser N, Richter SH. Improving reproducibility in animal research by splitting the study population into several ‘mini-experiments.’ Sci Rep. 2020;10(1):16579. doi:10.1038/s41598-020-73503-4
3. More than half of high-impact cancer lab studies could not be replicated in controversial analysis. Accessed December 11, 2024. https://www.science.org/content/article/more-half-high-impact-cancer-lab-studies-could-not-be-replicated-controversial-analysis
4. Pham LL, Watford SM, Pradeep P, et al. Variability in in vivo studies: Defining the upper limit of performance for predictions of systemic effect levels. Computational Toxicology. 2020;15:100126. doi:10.1016/j.comtox.2020.100126
5. Gosselin RD. Insufficient transparency of statistical reporting in preclinical research: a scoping review. Sci Rep. 2021;11(1):3335. doi:10.1038/s41598-021-83006-5
6. Steinmetz KL, Spack EG. The basics of preclinical drug development for neurodegenerative disease indications. BMC Neurol. 2009;9(Suppl 1):S2. doi:10.1186/1471-2377-9-S1-S2
7. Singh N, Vayer P, Tanwar S, Poyet JL, Tsaioun K, Villoutreix BO. Drug discovery and development: introduction to the general public and patient groups. Front Drug Discov. 2023;3:1201419. doi:10.3389/fddsv.2023.1201419
8. Tuntland T, Ethell B, Kosaka T, et al. Implementation of pharmacokinetic and pharmacodynamic strategies in early research phases of drug discovery and development at Novartis Institute of Biomedical Research. Front Pharmacol. 2014;5. doi:10.3389/fphar.2014.00174
9. Seeley RJ, MacDougald OA. Mice as experimental models for human physiology: when several degrees in housing temperature matter. Nat Metab. 2021;3(4):443-445. doi:10.1038/s42255-021-00372-0
10. Palarea‐Albaladejo J, McKendrick I. Best practice for the design and statistical analysis of animal studies. Veterinary Record. 2020;186(2):59-64. doi:10.1136/vr.m117
11. Hirst JA, Howick J, Aronson JK, et al. The need for randomization in animal trials: an overview of systematic reviews. PLoS One. 2014;9(6):e98856. doi:10.1371/journal.pone.0098856
12. Karp NA, Pearl EJ, Stringer EJ, Barkus C, Ulrichsen JC, Percie du Sert N. A qualitative study of the barriers to using blinding in in vivo experiments and suggestions for improvement. PLoS Biol. 2022;20(11):e3001873. doi:10.1371/journal.pbio.3001873
13. Bate S, Karp NA. A Common Control Group - Optimising the Experiment Design to Maximise Sensitivity. Peddada SD, ed. PLoS ONE. 2014;9(12):e114872. doi:10.1371/journal.pone.0114872
14. Commissioner O of the. FDA Announces Additional Steps to Modernize Clinical Trials. FDA. September 8, 2024. Accessed December 11, 2024. https://www.fda.gov/news-events/press-announcements/fda-announces-additional-steps-modernize-clinical-trials
15. Niazi S. The Coming of Age of AI/ML in Drug Discovery, Development, Clinical Testing, and Manufacturing: The FDA Perspectives. DDDT. 2023;Volume 17:2691-2725. doi:10.2147/DDDT.S424991
16. Zhou G, Rusnac DV, Park H, et al. An artificial intelligence accelerated virtual screening platform for drug discovery. Nat Commun. 2024;15(1):7761. doi:10.1038/s41467-024-52061-7
17. Wu K, Li X, Zhou Z, et al. Predicting pharmacodynamic effects through early drug discovery with artificial intelligence-physiologically based pharmacokinetic (AI-PBPK) modelling. Front Pharmacol. 2024;15:1330855. doi:10.3389/fphar.2024.1330855
18. Niarakis A, Laubenbacher R, An G, et al. Immune digital twins for complex human pathologies: applications, limitations, and challenges. npj Syst Biol Appl. 2024;10(1):141. doi:10.1038/s41540-024-00450-5
19. Gao S, Fang A, Huang Y, et al. Empowering biomedical discovery with AI agents. Cell. 2024;187(22):6125-6151. doi:10.1016/j.cell.2024.09.022
20. Sertkaya A, Beleche T, Jessup A, Sommers BD. Costs of Drug Development and Research and Development Intensity in the US, 2000-2018. JAMA Netw Open. 2024;7(6):e2415445. doi:10.1001/jamanetworkopen.2024.15445
21. Lowenstein P, Castro M. Uncertainty in the Translation of Preclinical Experiments to Clinical Trials. Why do Most Phase III Clinical Trials Fail? CGT. 2009;9(5):368-374. doi:10.2174/156652309789753392
22. Storey J, Gobbetti T, Olzinski A, Berridge BR. A Structured Approach to Optimizing Animal Model Selection for Human Translation: The Animal Model Quality Assessment. ILAR Journal. 2021;62(1-2):66-76. doi:10.1093/ilar/ilac004
23. Xu H, Shuttleworth KMJ. Medical artificial intelligence and the black box problem: a view based on the ethical principle of “do no harm.” Intelligent Medicine. 2024;4(1):52-57. doi:10.1016/j.imed.2023.08.001
AI Disclosure: Some of this content was generated with assistance from AI tools for copywriting.


.png)
.png)
.avif)
